Han J, Jin M, Zhang P, Liu J, Wang L, Wen D, Wu X,. Epidemiological link between exposure to poultry and all influenza A(H7N9) confirmed cases in Huzhou city, China, March to May 2013. Eurosurveillance, Volume 18, Issue 20, 16 May 2013
Since February 2013, a novel avian influenza A(H7N9) virus has led to an outbreak in the Yangtze River Delta Region and elsewhere in China [1,2]. As of 10 May 2013, it has resulted in 129 cases, including 31 deaths. Sporadic human infections by several H7 subtypes of influenza A viruses (e.g. H7N2, H7N3 and H7N7) had been reported previously in several countries in Europe and North America [3]. Apart from an influenza A(H7N7) outbreak in the Netherlands in 2003, infections with these H7 subtypes usually result in a mild, self-limiting illness [3]. In contrast, in the current influenza A (H7N9) outbreak, infection with the virus has resulted in severe and fatal respiratory disease [2,4] – the first time human infections have been seen for this virus [1]. The origin of the virus has been demonstrated to be associated with a reassortant event between three earlier avian influenza viruses [1,5]. Its genome comprises a haemagglutinin (HA) fragment from A(H7N3), a neuraminidase (NA) fragment from an earlier A(H7N9) virus and six internal genomic fragments from A(H9N2).
Two recent studies have provided compelling evidence that the novel A(H7N9) viruses from patients have a close genetic relationship with isolates from poultry [6,7], suggesting that the A(H7N9) virus may have spread to humans from poultry. However, preliminary epidemiological data showed that 18 of 77 confirmed cases did not have a history of contact with poultry [2]. Therefore, it remains to be determined whether there is a direct epidemiological link between exposure to poultry and human A(H7N9) virus infection.
Huzhou city, located in northern Zhejiang Province, China, is the geographical centre of the Yangtze River Delta (Figure 1). As of 10 May, 12 confirmed A(H7N9) cases have been reported in Huzhou city, accounting for about 9% (12/129) of all cases in China. There are two natural wetlands that provide habitats for over a 160 kinds of wild birds and, until the markets were closed, there had been an active live poultry business in Huzhou city. Therefore, we performed a detailed epidemiological study of the links between the confirmed cases and prior exposure to poultry.
Figure 1. Distribution of the influenza A(H7N9) confirmed cases and selected live poultry markets in Huzhou city, China, March–May 2013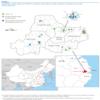
Data collection
A total of 12 persons were identified as influenza A(H7N9) confirmed cases, according to the definition in the national guidelines [8]. The infection was laboratory confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis [9].
A close contact was defined as a person who came within two metres of a confirmed case without the use of effective personal protective equipment (e.g. masks and gloves) during the presumed infectious period. The close contacts included, among others, the cases’ families and clinical staff (doctors and nurses) who had been in contact with the cases. All close contacts were traced and quarantined for seven days after their most recent exposure to a confirmed case.
Information on cases’ demographic characteristics, dates of symptom onset, exposure to poultry and/or other animals and/or visits to a live poultry market during the 10 days before symptom onset, as well as clinical signs and symptoms were collected using a standardised questionnaire and an open interview with the cases or their relatives when the cases were admitted to hospital. In our investigation, a ‘visit’ included only occasions in which a case either bought poultry, or had been close to or touched live poultry booths at a market.
To determine the source of the influenza A(H7N9) virus, we collected poultry faeces, waste (swab samples from culling benches) and sewage from the nine live poultry markets visited by the cases, for detection of A(H7N9) viral RNA by real-time RT-PCR.
In addition, samples from several surrounding live poultry markets (n=7) not visited by cases were also collected.
Data analysis
Demographic and clinical characteristics of influenza A(H7N9) cases in Huzhou city
As of 10 May 2013, 12 influenza A(H7N9) cases (four were male and eight female) were confirmed in Huzhou city (Table 1). As of 30 April, two had died, four had recovered fully, two were recovering and the other four remained critically ill (Figure 2). The median age was 60 years (range: 32–81) and most (n=9) were aged over 50 years.
Table 1. Demographic and exposure information of influenza A(H7N9) confirmed cases in Huzhou city, China, March–May 2013 (n=12)
Figure 2. Timeline of laboratory-confirmed influenza A(H7N9) cases in Huzhou city, China, March–May 2013 (n=12)
The first case developed symptoms on 29 March 2013; the infection was laboratory confirmed on 4 April [6]. In fact, another patient (Case 2) became ill earlier, on 12 March, but the infection was not laboratory confirmed until 8 April. The last two patients (Cases 11 and 12) both became ill on 17 April and were laboratory confirmed on 25 and 26 April, respectively. The initial symptoms were fever (axillary temperature greater than 37.5 °C) (n=7), cough (n=4), myalgia (n=4), chills (n=2), weakness (n=2), nasal obstruction and runny nose (n=1), expectoration (n=1), pruritic body rash (n=1), chest tightness (n=1) and nausea (n=1). Of the 12 cases, nine developed severe pneumonia and pulmonary dysfunction 2–10 days after symptom onset.
Of the 12 cases, 10 had chronic underlying conditions such as hypertension, bronchitis or heart disease, before infection. Three cases had low counts of white blood cells (between 1.7 x 109/L and 3.5 x 109/L); in another two, the count was high (12.7 x 109/L and 13.4 x 109/L), while the others were within the normal reference range (4–10 x 109/L). All but one case (with 3.4 mg/L) had high levels of high-sensitivity C-reactive protein (between 18.4 mg/L and >200 mg/L (i.e. exceeding the detection range); normal reference range: 0–10 mg/L).
All cases had a history of exposure to poultry before symptom onset
Nine of the 12 cases had visited nearby live poultry markets at least once (range: 1–10 times) during the 10 days before symptom onset (Table 1). Of these nine cases, four (Cases 4, 5, 6, and 8) had had direct contact with live poultry during this time. Although three patients had not visited poultry markets, they all had a history of direct contact with live poultry during the 10 days before symptom onset. Case 7 was exposed to live poultry as part of a government campaign to cull poultry at live poultry markets. Case 9 and her neighbour had purchased 12 chickens from a chicken vendor and had raised them in the same courtyard for about 20 days. Case 9 killed her seven chickens when she found that one of them had become ill. For Case 11, her husband purchased four live chickens from a market on 8 April and raised them at home. On 10 April, because the chickens developed an acute illness, the patient gave them antibiotics.
Influenza A(H7N9) viral RNA was detected in all poultry markets visited by cases
In total, nine live poultry markets were epidemiologically associated with the patients (Table 1, Figure 1). Therefore, we collected poultry faeces, waste and sewage from these markets, to test for the presence of A(H7N9) viral RNA. We also collected throat and anal swabs and faeces from the chickens raised by the neighbour of Case 9 and chicken faeces from the house of Case 11. Of the 135 samples obtained, 38 samples were positive. Of particular note, A(H7N9) viral RNA was detected in samples from all nine markets, as well as those from the courtyard of Case 9 and the house of Case 11.
In addition, we expanded our surveillance to seven other nearby live poultry markets that the cases had not visited. Of 75 samples tested, 23 were positive for A(H7N9) viral RNA.
We also collected throat swabs from the close contacts (n=339) of the 12 patients. Among 339 samples, none tested positive for A(H7N9) viral RNA, indicating no human-to-human transmission of the virus.
Discussion
Previous studies have suggested that several mutations in the HA might be involved in the acquisition of the ability of the A(H7N9) virus to infect humans [5-7,10], and genetic evidence indicates that poultry is the reservoir of the virus [6,7]. However, preliminary observations that not all patients have had a history of exposure to poultry raise the controversial issue of the source and transmission route of the A(H7N9) virus [2].
Our results provide epidemiological evidence to support the hypothesis that A(H7N9) virus-infected poultry are a transmission source. A total of 139 live poultry markets (including those tested) in the five districts or counties in Huzhou city were closed sequentially, from 11 April to 21 April (Table 2). As of 15 May, no new cases have been identified in Huzhou city (p<0.01). Although based on small case numbers, our findings support the view that poultry are a crucial transmission source and also indicate that closing live poultry markets in affected areas is an effective strategy to stop the outbreak.
Table 2. Effect of closure of live poultry markets in the five regions of Huzhou city, China, March–May 2013 
With respect to the absence of reported poultry exposures in some patients (n=18) in a previous study [2], we can suggest two possible explanations, arising from our findings: (i) some patients may have forgotten some details of their exposure history by the time the epidemiological investigation was carried out; or (ii) some patients may have been unable to provide timely and reliable information due to their serious clinical conditions. It may therefore be possible that patients with no documented exposure may have in fact been exposed to poultry.
We tested 339 throat swabs from the cases’ close contacts, but none tested positive for the A(H7N9) viral RNA, suggesting that these patients did not spread the virus to their close contacts. Although throat swabs may not be as often positive as deep sputum samples [7,11], we did not collect sputum samples from these close contacts because they had no obvious symptoms. Most patients (n=9) were aged 50 years or older, consistent with the nationwide data (78/107) [4]. Distinct from the nationwide data, however, two thirds (8/12) of the cases in Huzhou city were female (nationwide data: 32/106). This could possibly be due to the fact that in Huzhou city, housewives are mainly responsible for buying food, such as meat or vegetables, in local markets. It should also be borne in mind that most of the cases (n=10) had chronic underlying conditions. Whether an individual’s health status is associated with susceptibility to A(H7N9) virus infection remains to be proved.
Although an earlier study found that some live poultry markets tested positive, only a few poultry vendors (n=4) were found to be infected with the virus [2]. Why most vendors remained infection-free despite extremely frequent exposure to infected poultry is also unclear. Whether there is some pre-existing cross-reactive immunity, which enhances the susceptibility of patients to A(H7N9) virus infection [4] or prevents poultry vendors from infection needs to be determined
See Also:
Latest articles in those days:
- Phylogenetic Analysis of Highly Pathogenic Avian Influenza H7 Viruses in Australia and New Zealand Suggests Local Viral Evolution 17 hours ago
- AI-Powered Identification of Human Cell Surface Protein Interactors of the Hemagglutinin Glycoprotein of High-Pandemic-Risk H5N1 Influenza Virus 17 hours ago
- Seasonal Influenza Vaccination Uptake and Intentions Among Nursing Students in Hong Kong 17 hours ago
- Intranasal Mosaic H1N1 Live Attenuated Influenza Vaccine Elicits Broad Cross-Reactive Immunity and Protection Against Group 1 and 2 Influenza A Viruses 17 hours ago
- Changing Landscape of Pediatric Influenza in Northern Mexico: A Comparative Clinical and Virological Study 17 hours ago
[Go Top] [Close Window]


