Update: Influenza Activity United States, 2004--05 Season
submited by kickingbird at Mar, 4, 2005 8:40 AM from US CDC
Influenza activity has increased steadily in the United States since late December and, as of February 19, might not have peaked. Laboratory-confirmed influenza infections have been reported from all 50 states. This report summarizes influenza activity during October 3, 2004--February 19, 2005*.
Influenza Viral Surveillance and Characterization
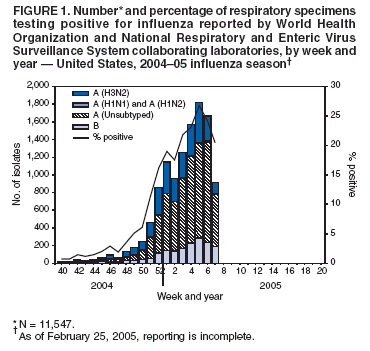
During October 3--February 19, World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories in the United States tested 83,753 respiratory specimens for influenza viruses; 11,547 (13.8%) were positive. The weekly percentage of specimens that tested positive for influenza ranged from 0.7% to 26.7% (Figure 1) and first exceeded 10.0% during the week ending December 25 (week 51). During the 2001--02, 2002--03, and 2003--04 influenza seasons, peak percentages of specimens that tested positive for influenza ranged from 24.9% to 34.7% (CDC, unpublished data, 2004). During January 30--February 19, by region, the percentage of specimens that tested positive for influenza ranged from 8.9% in the Pacific region to 42.7% in the East North Central region?/SUP>.
Of the 11,547 influenza viruses identified since October 3, a total of 9,773 (84.6%) were influenza A viruses, and 1,774 (15.4%) were influenza B viruses. Among the influenza A viruses, 3,001 (30.7%) were subtyped; 2,990 (99.6%) were influenza A (H3N2), and 11 (0.4%) were influenza A (H1)?/SUP>. In the Mid-Atlantic and New England regions, 95% and 94% of viruses reported were influenza type A, respectively. In the remaining seven surveillance regions, the proportion of influenza A viruses ranged from 60% in the Pacific region to 88% in the East South Central region.
Using hemagglutination-inhibition tests with post-infection ferret serum, CDC has antigenically characterized 320 influenza viruses collected by U.S. laboratories since October 1, 2004. Of these, 228 (71.3%) were influenza A (H3N2) viruses, two (<1%) were influenza A (H1N1) viruses, and 90 (28.1%) were influenza B viruses. Of the 228 influenza A (H3N2) isolates, 125 (54.8%) were A/Fujian/411/2002-like (H3N2), the influenza A (H3N2) strain recommended for the 200405 influenza vaccine?/SUP>, and 103 (45.2%) were antigenically similar to A/California/7/2004 (H3N2), a recently characterized drift variant of A/Fujian/411/2002-like (H3N2) viruses. Current influenza B viruses fall into one of two antigenically and genetically distinct lineages represented by B/Yamagata/16/88 and B/Victoria/2/87 viruses (1). Of the 90 influenza B viruses, 66 (73.3%) were similar to B/Shanghai/361/2002-like viruses (from the B/Yamagata/16/88 lineage), the influenza B strain recommended for the 200405 influenza vaccine, five (5.6%) had reduced titers to B/Shanghai/361/2002 using ferret antisera, and 19 (21.1%) belonged to the B/Victoria/2/87 lineage.
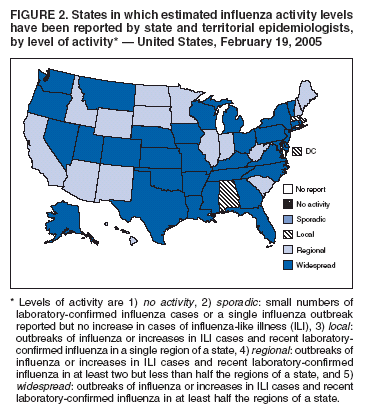
For the week ending February 19, 2005, a total of 33 states reported widespread influenza activity**; 15 states reported regional activity; and two states, New York City, and the District of Columbia reported local influenza activity (Figure 2). Since the week ending October 9, 2004, a total of 38 states and New York City have reported widespread influenza activity for at least 1 week.
During the weeks ending October 9--February 19, weekly percentages of patient visits for influenza-like illness (ILI)唵 reported by approximately 1,500 U.S. sentinel providers from all 50 states ranged from 1.0% to 5.7%. During the week ending February 19, a total of 5.7% of patient visits were for ILI. This was the sixth consecutive week that the percentage of visits for ILI exceeded the national baseline of 2.5%Ё. During the 2001--02, 2002--03, and 2003--04 influenza seasons, national weekly peak percentages of patient visits for ILI ranged from 3.2% to 7.6% (CDC, unpublished data, 2004).
The New Vaccine Surveillance Network (NVSN) provides population-based estimates of laboratory-confirmed influenza hospitalization rates for children aged <5 years residing in three cities (Cincinnati, Ohio; Nashville, Tennessee; and Rochester, New York). Children admitted to NVSN hospitals with fever or respiratory symptoms are prospectively enrolled, and respiratory samples are collected and tested by viral culture and reverse transcriptase-polymerase chain reaction (PCR). During October 3, 2004--February 5, 2005, the preliminary hospitalization rate was 2.0 per 10,000 children. During 20002--004, the influenza (October to mid-May) hospitalization rates ranged from 3.7 per 10,000 children (2002--2003) to 12.0 (2003--2004). The Emerging Infections Programs (EIP) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in persons aged <18 years in 11 metropolitan areas (San Francisco, California; Denver, Colorado; New Haven, Connecticut; Atlanta, Georgia; Baltimore, Maryland; Minneapolis/St. Paul, Minnesota; Albuquerque, New Mexico; Albany, New York; Rochester, New York; Portland, Oregon; and Nashville, Tennessee). Hospital laboratory and admission databases and infection-control logs are reviewed to identify children with a positive influenza test result (i.e., culture, direct or indirect fluorescent antibody assays, PCR, or a rapid test) from testing conducted as a part of their routine care. During October 1--February 5, the preliminary hospitalization rates for children aged 0--4 years and 5--17 years were 0.81 and 0.11 per 10,000, respectively (combined hospitalization rate: 0.35). The final 2003--04 influenza season EIP hospitalization rates were 7.8 per 10,000 children aged 0--4 years and 0.8 per 10,000 children aged 5--17 years.
During the week ending February 19, a total of 8.5% of deaths reported through the 122 Cities Mortality Reporting System were attributed to pneumonia and influenza (P&I), which is above the epidemic threshold of 8.2%抖 for that week. The percentage of P&I deaths exceeded the epidemic threshold for 3 nonconsecutive weeks during October 3--February 19 but otherwise has remained below threshold. In October 2004, pediatric deaths associated with laboratory-confirmed influenza infection became a nationally notifiable condition. As of February 19, nine states (California, Georgia, Maine, Massachusetts, Mississippi, New Jersey, Ohio, Pennsylvania, and Vermont) had reported nine pediatric deaths to CDC; all deaths occurred during January and February. Reported by: J Tang, MPH, L Brammer, MPH, K Teates, MPH, S Harper, MD, K Fukuda, MD, A Klimov, PhD, T Wallis, MS, N Cox, PhD, WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, Div of Viral and Rickettsial Diseases; R Seither, MPH, M Iwane, PhD, Epidemiology and Surveillance Div; J Copeland, MS, National Immunization Program, CDC.
Influenza activity was low in the United States from October through mid-December but steadily increased during January and February and might not have peaked. In the United States, influenza activity typically peaks during December--March (2) and, in 16 of the preceding 27 seasons, has peaked during February or later. During the 2003--04 influenza season, 153 pediatric deaths associated with influenza infection were reported from 40 states, whereas only nine such deaths have been reported so far this season. However, numerous influenza outbreaks have been reported in long-termcare facilities and among school children, and the number of pediatric deaths associated with laboratory-confirmed influenza is expected to increase before the end of this season. The viruses circulating this year include both influenza A and B viruses, but influenza A viruses have predominated, and most have been subtyped as influenza A (H3N2) viruses. Most of the influenza A (H3N2) viruses reported earlier in the season were antigenically similar to the influenza A (H3N2) component of the 2004--05 vaccine (A/Fujian/411/2002-like virus). However, since mid-January, an increasing proportion of influenza A (H3N2) viruses have been reported to be similar to A/California/7/2004, a recent reference strain that is related to A/Fujian/411/2002 but is antigenically distinguishable. Antibodies produced against A/Fujian/411/2002-like viruses cross-react with A/California7/2004-like viruses but at a lower level, and, because of this, effectiveness of the 2004--05 vaccine could be reduced against A/California/7/2004-like viruses. Antiviral medications are useful for early treatment of influenza and as an adjunct to influenza vaccination for influenza prevention and control. They should be considered when treating persons with suspected influenza regardless of vaccination status during periods of community influenza activity. Influenza antiviral drugs differ in approved age groups, recommended dosages, routes of administration, adverse effects, development of antiviral resistance, and cost. When administered within 48 hours of symptom onset, antiviral treatment of influenza can reduce the duration of illness by approximately 1 day in healthy adults (3). Four prescription antiviral medications (amantadine, rimantadine, oseltamivir, and zanamivir) are approved for treatment of influenza A virus infections. Oseltamivir and zanamivir also are approved for treatment of influenza B virus infections. Antiviral chemoprophylaxis is approximately 70%--90% effective in preventing illness in healthy adults (3). Amantadine, rimantadine, and oseltamivir are approved for chemoprophylaxis of influenza A virus infections; only oseltamivir is approved for chemoprophylaxis of influenza B virus infections. Physicians should consult package inserts of antiviral drugs for information on approved age groups, dosing, and adverse effects. Influenza surveillance reports for the United States are published weekly during October--May and are available at http://www.cdc.gov/flu/weekly or through the CDC voice (888-232-3228) and fax (888-232-3299, document number 361100) information systems. Acknowledgments The findings in this report are based, in part, on data contributed by participating state and territorial health departments and state public health laboratories, WHO collaborating laboratories, National Respiratory and Enteric Virus Surveillance System collaborating laboratories, the U.S. Influenza Sentinel Provider Surveillance System, the New Vaccine Surveillance Network, the Emerging Infections Program, and the 122 Cities Mortality Reporting System.
* As of February 25, 2005, reporting is incomplete. ?/SUP> Surveillance regions: New England: Connecticut, Maine, Massachusetts, New Hampshire, Vermont, and Rhode Island; Mid-Atlantic: New Jersey, New York City, Pennsylvania, and Upstate New York; East North Central: Illinois, Indiana, Michigan, Ohio, and Wisconsin; West North Central: Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota; South Atlantic: Delaware, District of Columbia, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, and West Virginia; East South Central: Alabama, Kentucky, Mississippi, and Tennessee; West South Central: Arkansas, Louisiana, Oklahoma, and Texas; Mountain: Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming; and Pacific: Alaska, California, Hawaii, Oregon, and Washington. ?/SUP> Includes both the A (H1N1) and A (H1N2) influenza virus sybtypes. ?/SUP> The A/Fujian/411/2002-like virus used by U.S. vaccine manufacturers was A/Wyoming/03/2003, an antigenically equivalent virus appropriate for vaccine production. ** Levels of activity are 1) no activity, 2) sporadic: small numbers of laboratory-confirmed influenza cases or a single influenza outbreak reported but no increase in cases of influenza-like illness (ILI), 3) local: outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in a single region of a state, 4) regional: outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in at least two but less than half the regions of a state, and 5) widespread: outbreaks of influenza or increases in ILI cases and recent laboratory-confirmed influenza in at least half the regions of a state. 唵 Temperature of >100.0?/SUP>F (>37.8?/SUP>C) and either cough or sore throat in the absence of a known cause. Ё The national baseline was calculated as the mean weighted percentage of visits for ILI during noninfluenza weeks, plus two standard deviations. Wide variability in regional data precludes calculating region-specific baselines; applying the national baseline to regional data is inappropriate. 抖 The expected seasonal baseline proportion of P&I deaths reported by the 122 Cities Mortality Reporting System is projected by using a robust cyclical regression procedure in which a periodic regression model is applied to the observed percentage of deaths from P&I during the previous 5 years. The epidemic threshold is 1.645 standard deviations above the seasonal baseline. Influenza Activity Levels Reported by State and Territorial Epidemiologists
Patient Visits for Influenza-Like Illness
Pediatric Hospitalizations Associated with Laboratory-Confirmed Influenza Infection
Influenza-Associated Mortality Surveillance
Editorial Note:
References
- UK: Bird flu (avian influenza): latest situation 18 hours ago
- Canada: Highly pathogenic avian influenza in British Columbia, December 17, 2025 2 days ago
- Canada: Highly pathogenic avian influenza in Ontario, December 15, 2025 3 days ago
- Updates of Avian Influenza situation by FAO/EMPRES-AH (September 2025 - December 2025) 4 days ago
- Canada: Highly pathogenic avian influenza in Saskatchewan, December 12, 2025 4 days ago
[Go Top] [Close Window]