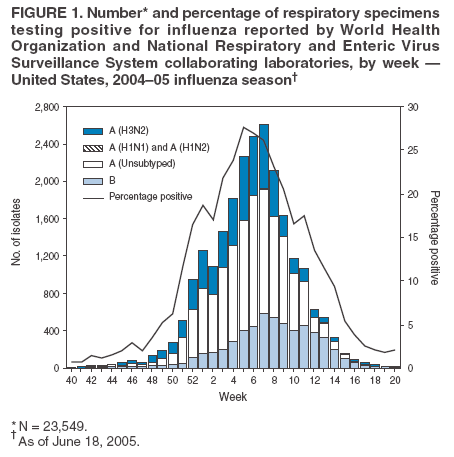
State-Specific Activity Levels
Influenza activity, as reported by state and territorial epidemiologists, peaked during the week ending February 19, 2005 (week 7), when 30 states reported widespread influenza activity and 13 states reported regional activity.?/SUP> A total of 42 states and New York City reported widespread influenza activity for at least 1 week. No states reported widespread, regional, or local influenza activity during the weeks ending May 7--21, 2005 (weeks 18--20). The peak number of states reporting widespread or regional activity during the previous three seasons ranged from 35 to 50 states (1; CDC, unpublished data, 2005).
Laboratory-confirmed, influenza-associated, pediatric hospitalizations are monitored in two population-based surveillance networks: the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN). During October 1, 2004--April 30, 2005,** the preliminary influenza-associated hospitalization rates for children aged 0--4 years reported by NVSN and EIP were 7.0 and 3.1 per 10,000, respectively. EIP also monitors hospitalizations in children aged 5--17 years; the preliminary influenza-associated hospitalization rate for this age group was 0.6 per 10,000. The overall hospitalization rate reported by EIP for children aged 0--17 years was 1.3 per 10,000. During 2000--2004, the end-of-season hospitalization rate for NVSN ranged from 3.7 (2002--03) to 12.0 (2003--04) per 10,000 children. The 2003--04 end-of-season hospitalization rate for EIP was 8.9 per 10,000 children aged 0--4 years and 0.8 per 10,000 for children aged 5--17 years. The difference in rates between NVSN and EIP is likely attributable to different case-finding methods and the different populations monitored.唵
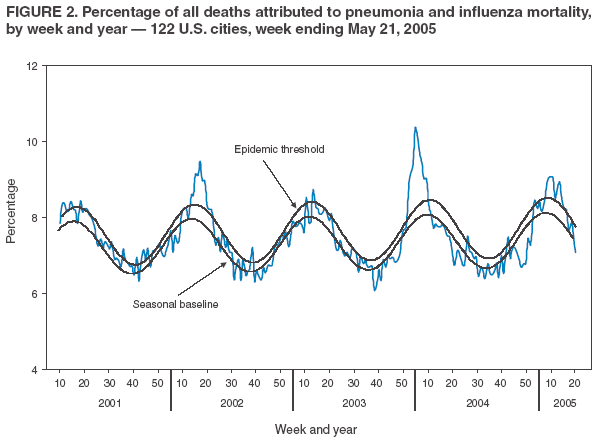
As measured by the 122 Cities Mortality Reporting System, the percentage of deaths in the United States attributed to pneumonia and influenza (P&I) exceeded the epidemic thresholdЁ during 8 consecutive weeks ending February 14--April 9, 2005, and peaked at 8.9% during the week ending March 5, 2005 (Figure 2). The percentage of P&I deaths remained below the threshold through the weeks ending April 30--May 21, 2005. During the previous three influenza seasons, the peak percentage of P&I deaths ranged from 8.5% to 10.4% (1; CDC, unpublished data, 2005).
In October 2004, pediatric deaths (i.e., deaths in children aged <18 years) associated with laboratory-confirmed influenza infection became a nationally notifiable condition. For the 2004--05 influenza season, 36 pediatric deaths have been reported to CDC from 16 states (California, Colorado, Florida, Georgia, Iowa, Maine, Maryland, Massachusetts, Michigan, Mississippi, Nevada, New Jersey, New York, Ohio, Pennsylvania, and Vermont) and New York City; all deaths were reported during January--June 2005.
During October 2004--May 2005, influenza A viruses circulated widely worldwide. Influenza A (H3N2) viruses predominated in most countries, whereas influenza A (H1) and B viruses circulated at low levels in most parts of the world. Influenza A (H3N2) viruses predominated and were associated with outbreaks in Asia (Hong Kong, Indonesia, Israel, and South Korea), Europe (Belgium, Finland, France, Germany, Italy, Latvia, Norway, Portugal, Romania, the Russian Federation, Spain, Sweden, Switzerland, Turkey, Ukraine, and the United Kingdom), and North America (Canada). Influenza A (H3N2) viruses also were reported in Africa (Egypt, Madagascar, Morocco, Senegal, South Africa, and Tunisia), Asia (China, India, Iraq, Iran, Japan, Kyrgyzstan, Malaysia, the Philippines, Singapore, Taiwan, and Thailand), Europe (Austria, Belarus, Bulgaria, Czech Republic, Denmark, Greece, Hungary, Iceland, Ireland, the Netherlands, Poland, Serbia and Montenegro, and Slovenia), South America and the Caribbean (Argentina, Brazil, Chile, Dominica, Guyana, Peru, Saint Lucia, and Venezuela), North America (Canada and Mexico), and Oceania (Australia, Guam, New Caledonia, and New Zealand). Influenza A (H1) viruses circulated at low levels in most parts of the world. Influenza A (H1) viruses were isolated in Africa (Senegal, South Africa, and Tunisia), Asia (China, Hong Kong, Indonesia, Iran, Israel, Japan, Kazakhstan, Kyrgyzstan, Malaysia, Singapore, South Korea, Taiwan, and Thailand), Europe (Austria, Belgium, Bulgaria, Czech Republic, Denmark, France, Germany, Greece, Ireland, Italy, Latvia, Norway, Poland, Portugal, Romania, Russian Federation, Slovakia, Sweden, Switzerland, Turkey, Ukraine, and the United Kingdom), and South America (Brazil and Peru). Influenza B viruses were reported in association with outbreaks in Asia (China, Hong Kong, Japan, and Taiwan) and Europe (Denmark, Ireland, and the Netherlands). Influenza B viruses also were isolated in Africa (Egypt, Madagascar, Morocco, Senegal, South Africa, and Tunisia), Asia (Bangladesh, India, Indonesia, Israel, Malaysia, South Korea, Singapore, and Thailand), the Caribbean (Jamaica and Saint Lucia), Europe (Austria, Belarus, Belgium, Czech Republic, Finland, France, Germany, Greece, Iceland, Italy, Latvia, Norway, Portugal, Romania, Russia, Spain, Sweden, Switzerland, Turkey, Ukraine, and the United Kingdom), South America (Argentina, Brazil, Chile, Colombia, Guyana, Paraguay, Peru, and Uruguay), North America (Canada and Mexico), and Oceania (Australia, New Caledonia, and New Zealand).
During January 2004--June 28, 2005, a total of 108 human cases of avian influenza A (H5N1) infection resulting in 54 deaths were reported in Vietnam (87 cases and 38 deaths), Thailand (17 cases and 12 deaths), and Cambodia (four cases and four deaths) (2). From mid-December 2004 through June 28, 2005, a total of 60 cases (18 deaths) were reported in Vietnam, and four cases (four deaths) were reported in Cambodia (2). Reported by: WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza. R Dhara, MPH, K Teates, MPH, L Brammer, MPH, T Wallis, MS, A Postema, MPH, T Uyeki, MD, A Klimov, PhD, K Fukuda, MD, N Cox, PhD, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC.
During the 2004--05 influenza season, influenza A (H3N2) viruses predominated in most countries in Asia, Europe, and North America, but influenza A (H1) and B viruses were also identified. In the United States, influenza activity peaked in February and was less severe than during the previous season. Human infections with avian influenza A (H5N1) viruses continue to be identified in Southeast Asia. To date, the majority of cases have been associated with direct exposure to A (H5N1)-infected poultry. Probable, limited, person-to-person transmission of A (H5N1) viruses during 2004 occurred in Thailand (3) and is one of several possible explanations for the observed increase in clusters of A (H5N1) cases in northern Vietnam during 2005 (4). Limited, person-to-person transmission of A (H5N1) was also identified during the 1997 outbreak in Hong Kong (5). However, efficient, sustained, person-to-person transmission of influenza A (H5N1) viruses has not been reported to date. Genetic analysis of influenza A (H5N1) viruses isolated from humans in 2004 and 2005 revealed that all genes were of avian origin. CDC continues to recommend enhanced surveillance for influenza A (H5N1) infection among travelers with severe unexplained respiratory illness returning from A (H5N1)-affected countries. Additional information is available at http:// www.phppo.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00221. Additional information on influenza, including avian influenza, is available at http://www.cdc.gov/flu. Updates on human infections with avian influenza are available from the World Health Organization at http://www.who.int/csr/disease/avian_influenza/en. Acknowledgments This report is based on data contributed by participating state and territorial health departments and state public health laboratories, WHO collaborating laboratories, National Respiratory and Enteric Virus Surveillance System collaborating laboratories, the U.S. Influenza Sentinel Provider Surveillance System, the New Vaccine Surveillance Network, the Emerging Infections Program, and the 122 Cities Mortality Reporting System. WHO National Influenza Centers, WHO Global Influenza Programme, Geneva, Switzerland. I Gust, MD, A Hampson, WHO Collaborating Center for Reference and Research on Influenza, Parkville, Australia. A Hay, PhD, WHO Collaborating Center for Reference and Research on Influenza, National Institute for Medical Research, London, England. M Tashiro, MD, WHO Collaborating Center for Reference and Research on Influenza, National Institute of Infectious Diseases, Tokyo, Japan. Bur of Epidemiology and Field Epidemiology Training Program, Thai Ministry of Public Health. National Center for Public Health Informatics; National Immunization Program, CDC.
* Includes both the A (H1N1) and A (H1N2) influenza virus types. ?/SUP> Defined as temperature of >100.0?/SUP> F (>37.8?/SUP> C) and either cough or sore throat in the absence of a known cause other than influenza. ?/SUP> The national baseline was calculated as the mean percentage of patient visits for ILI during non-influenza weeks plus two standard deviations. Wide variability in regional data precludes calculating region-specific baselines and makes it inappropriate to apply the national baseline to regional data. National and regional percentages of patient visits for ILI are weighted on the basis of state population. ?/SUP> Levels of activity are 1) no activity; 2) sporadic: isolated laboratory-confirmed influenza cases or laboratory-confirmed outbreak in one institution, with no increase in activity; 3) local: increased ILI in one region, or at least two institutional outbreaks (ILI or laboratory-confirmed influenza) in one region; virus activity no greater than sporadic in other regions; 4) regional: increased ILI activity or outbreaks (ILI or laboratory-confirmed influenza) in at least two but fewer than half of the regions in the state; and 5) widespread: increased ILI activity or outbreaks (ILI or laboratory-confirmed influenza) in at least half the regions in the state. ** Active prospective surveillance in EIP and NVSN for the 2004--05 influenza season ended as of April 30, 2005. 唵 NVSN provides population-based estimates of laboratory-confirmed influenza hospitalization rates in children aged <5 years admitted to NVSN hospitals with fever or respiratory symptoms. Children are prospectively enrolled, and respiratory samples are collected and tested by viral culture and reverse transcriptase-polymerase chain reaction (PCR). EIP conducts surveillance for laboratory-confirmed, influenza-related hospitalizations in person aged <18 years. Hospital laboratory and admission databases and infection-control logs are reviewed to identify children with a positive influenza test result (i.e., culture, direct or indirect fluorescent antibody assays, PCR, or a rapid test) from testing conducted as a part of their routine care. Ё The expected seasonal baseline proportion of P&I deaths reported by the 122 Cities Mortality Reporting System is projected by using a robust regression procedure in which a periodic regression model is applied to the observed percentage of deaths from P&I during the previous 5 years. The epidemic threshold is 1.654 standard deviations above the seasonal baseline.
Influenza-Associated Pediatric Hospitalizations
Pneumonia and Influenza-Related Mortality
Influenza-Associated Pediatric Mortality
Worldwide Influenza Activity
Human Infections with Avian Influenza A (H5N1) Viruses
Editorial Note:
References